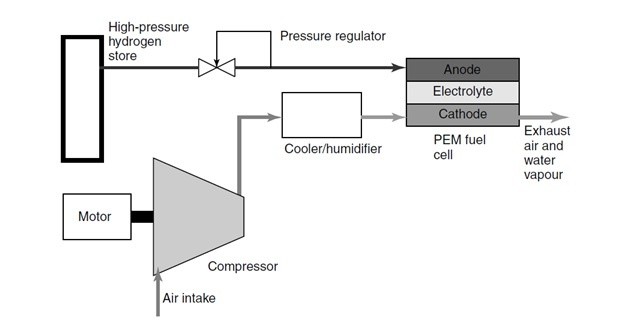
Could a high-pressure direct-injection hydrogen engine replace the turbodiesel?
Having fallen from grace, the once iconic diesel power unit appears to have run its course. Even cities, such as Paris, that once incentivized the use of diesel are now calling for OEMs to stop production by 2025. Although this is highly unlikely to happen, it is an expression of the world’s concerns over global warming and air pollution in general.
To meet ever tightening emissions regulations OEMs are studying new and often untried forms of propulsion: Everything from full electrification to hybrids and even hydrogen fuel cells are being tested as possible solutions.
Hydrogen in particular is piquing the interest of researchers around the world – it’s hailed as a clean burning fuel that could very well end up powering the transport of the future.
The difference between hydrogen and conventional hydrocarbons lies in its wide stoichiometric range from 4 to 75 percent by volume hydrogen to air, and under ideal conditions the burning velocity of hydrogen can reach some hundred meters per second. These characteristics make it highly efficient when burning lean mixtures with low NOx emissions.
Forty years of hydrogen injection
Hydrogen injection has been around since the 1970s and works by injecting hydrogen into a modified, internal combustion engine, which allows the engine to burn cleaner with more power and lower emissions.
Earlier low pressure systems, which are still in use today, injected the hydrogen into the air prior to entering the combustion chamber. But with hydrogen burning 10 times faster than diesel and, once mixed with the diesel in the combustion chamber, increasing the burn rate several problems have been experienced. The most significant being:
- Light-back of the gas in the manifold
- Preignition and/or autoignition.
The best way to overcome these problems is to fit a high-pressure direct injection system that provides fuel injection late in the compression stroke.
Optimizing the combustion process through accurate pressure measurement
In order to do this the injection needs to be accurately mapped to the engine. This can only be accomplished through gathering test data regarding temperature (manifold, EGT and coolant), pressure (cylinder/ boost, line and injector), the turbulence in the manifold and combustion chamber, and the gas composition.
The mixture formation, the ignition and the burning processes are commonly studied through two different sets of experiments. The aim of the first experiment is to obtain information about the highly transient concentration and distribution of hydrogen during the injection process.
During this test a Laser-Induced Fluorescence (LIF) on tracer molecules is used as the primary measurement technique to study the behavior of the hydrogen under compression and ignition. Using a constant volume combustion chamber (CVCC) with the same dimensions as the actual C.I. engine, implying that the volume in the CVCC equals the volume in the cylinder at the top dead center, pressurized hydrogen is injected into the cold pressurized air through a hydraulically controlled needle valve.
Using high quality pressure sensors, the effect of various injection pressures on the combustion process can be studied. By observing the behavior and volume of unburned gas, the time taken to optimize the injection pressure for a specific number and position of injector nozzle holes and also the injection direction is drastically reduced.
And using unique software the ignition delay, which is dependent on the temperature and the concentration of hydrogen in air at a given pressure can be determined. Once again, it’s important that the pressure readings are accurately recorded, across a range of pressures that vary between 10 to 30 MPa.
Furthermore, this method allows for the definition of areas of the injection jet where self-ignition conditions exist, which is useful for the development of an optimized injection system for engines to be converted from diesel fuel to hydrogen.
In recent tests carried out by a premium brand OEM,the optimized high pressure hydrogen injected engine showed a promising increase in specific power while reducing fuel consumption and achieving 42% efficiency – values that match the best turbodiesel engines.
Based on the findings it would certainly appear as if work carried out on optimizing the pressure of these 30 MPa systems may in fact offer another source of clean energy for future transport.